The so called "blackbody radiation" is a very interesting phenomenon: every object radiates (and absorbs) electromagnetic waves. The spectrum of this radiation is not dependent on the chemical composition of the matter but it's only determined by its absolute temperature T. The term blackbody comes from a theoretical model of an object absorbing all incident radiation that is used to develop the quantum mechanic equations. It turns out that all objects behaves like blackbodies, regardless if they are actually black or not.
At ambient temperature the majority of the emitted spectrum is in the long wave infrared which is not visible. As the temperature rises, the spectrum shifts towards shorter wavelengths. At temperatures around 900 K, part of the radiation becomes visible since wavelengths in the 700 nm region are present and the object start to appear "red hot".
If you think of a blacksmith working a piece of hot iron, the iron glows red because its temperature is around 1'000 K, but the charcoal in the furnace glows the same color because it's at about the same temperature, even if carbon and iron are chemically very different.
At higher temperatures the color of the radiation will tend to yellow, white and white-blue, roughly according to the table below. Please keep in mind that color perception is subjective and different authors report slightly different colors.
| 1'000 K | Red |
| 1'500 K | Reddish orange |
| 2'000 K | Yellowish orange |
| 2'800 K | Yellow |
| 3'500 K | Yellowish white |
| 4'500 K | Warm white |
| 5'500 K | White |
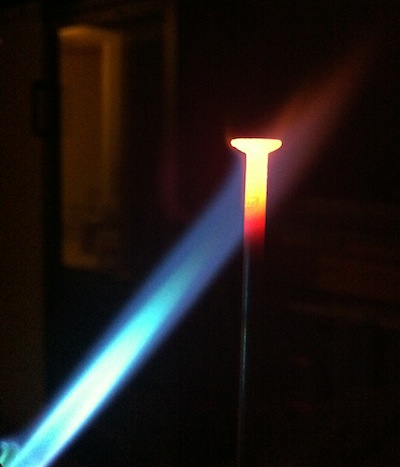
The picture below shows a nail glowing red hot when heated with a propane torch: one can clearly see the hottest part of the nail glowing yellow, the part that is just outside the flame glowing red and the rest being black because normal cameras cannot see infrared radiation. The nice blue color of the flame is not due to blackbody radiation: the temperature of a propane torch is around 3'000 K so the flame should glow yellow, but the chemical reaction taking place emits a much stronger blue radiation masking the faint yellow glow. This blue color depends on the chemicals used and different gasses will burn with different flame colors.

The spectrum of blackbody radiation has a typical bell shape and the emitted energy (integral of the curve) is proportional to the forth power of the absolute temperature (T4): hotter bodies radiate a lot more. The following plot shows the spectrum for temperatures from 273 K (0 °C) to 453 K (180 °C) in 20 °C steps.
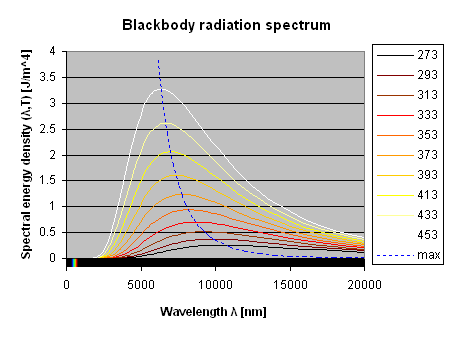
Back body radiation spectrum at 273, 293, 313, 333, 373, 393, 413, 433 and 453 K
As one can see, the majority of the radiation is in the long wave infrared, well beyond 5 μm. Almost nothing is emitted in the visible spectrum (roughly 400 to 700 nm). If our eyes could see wavelengths around 10 μm, there would be no such thing as darkness, since all objects at ambient temperature strongly emits and absorbs in this range.
The spectrum is described by Plank's equation:
.gif)
Where:
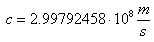
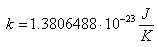
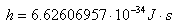
The following plot shows the same equation plotted for temperatures from 1'000 K (727 °C) to 3'250 K (2'977 °C) in 250 °C steps. Higher temperatures have been plotted on a separate figure than the previous one because of the T4 dependency: they are so much stronger that it would be difficult to appreciate everything together.
Back body radiation spectrum at 1000, 1250, 1500, 1750, 2000, 2250, 2500, 2750, 3000 and 3250 K.
Again, the majority of the radiation is still in the invisible infrared, but now a significant portion is in the visible range and the glow can be observed.
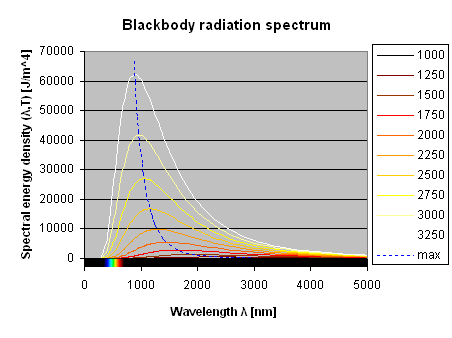
To better appreciate the color of a red-hot object as a function of its temperature, the figure below plots a zoom of the same Plank equation showing part of the visible spectrum and part of the near infrared.
Backbody radiation in the visible spectrum at 800, 850, 900, 950, 1000, 1050, 1100, 1150, 1200 and 1250 K.
As one can see, as the temperature increases, the portion of short wavelengths emitted increases as well. At 800 K, only some red wavelengths can be seen, but at higher temperatures orange and yellow start to appear and at 1'250 K there is also some green and cyan. The perceived color changes from deep red to bright red, orange, yellow and white as the spectrum fills up with all the colors.
Common hot bodies have maximum temperature below 3'500 K, the hottest being probably filament (halogen) light bulbs; they emit a yellowish white light. But there is an exception: the sun has a much hotter surface temperature, around 5'800 K. Sunlight spectrum is similar to a blackbody of roughly the same temperature and we see it as white light.
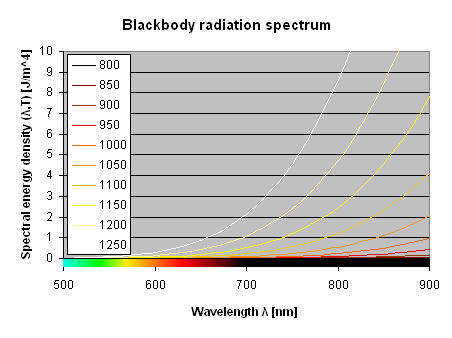
The wavelength of the peak of the blackbody radiation is proportional to 1/T and is called "Wien's shift" or "Wien's displacement law". In other terms, the hotter the body, the shorter the wavelength. The Wien's equation is given below:
![lambda_max=2.89776829E6[nm*K]/T](wiens.gif)
When plotted, it gives the following hyperbola:
Peak wavelength of blackbody radiation as a function of temperature.
This is the curve that is plotted as a blue dotted line over the bell shaped blackbody spectra shown above.
Since hot bodies are very often used as light sources color temperature is used to describe the color of the light. For example the sun, a filament light bulb, a candle, and many other light sources can be considered as "black body" radiators.
Color temperature in photography is simply measured by comparing the blue and red components of the light without exactly measuring its spectrum, but it's still expressed in Kelvins and values are very similar to the temperature of a black body.
The following table gives a few examples of the color temperature of some light sources:
| 1'500 K | Candlelight |
| 2'700 K | Incandescent lamp |
| 3'200 K | Sunrise / sunset |
| 3'400 K | Halogen incandescent lamp |
| 5'500 K | Sunny daylight around noon |
| 6'000 K | Electronic photo flash |
| 7'000 K | Overcast sky |
| 10'000 K | Blue sky |
Color temperature doesn't necessary refers to a hot body: the blue sky has a very high color temperature because of the blue color of its light, but this is just because the red and yellow light has been filtered out, the sky is physically very cold. Color temperature should be interpreted as the temperature of a body that would generate a very similar color of light.
Please remarks that hot bodies have cold color temperatures and vice-versa. This is because we think red objects as hot because this reminds us of fire and flames and we think at blue objects as cold because they remind us of water and ice. Blackbody radiation is the other way around: objects glowing blue-white are much much hotter than red glowing ones.
Blackbody radiation and color temperature have been briefly described. The phenomena involved are quite broad and involve a lot of physics: for more details I strongly suggest consulting the books reported in the bibliography section.
| [1] | Paul A. Tipler. College Physics. Worth Publishers Inc., 1987, section 29.1. |
| [2] | Richard P. Feynman, Robert B. Leighton and Matthew Sands. Lectures on Physics. The Definitive Edition, Volume III, Addison–Wesley, 2006, section 4-5. |
| [3] | A. Daeschler, G. Camponovo. Elettrotecnica. Edizioni Casagrande, Bellinzona, 1974, sezione 11.1.3. |
| Home | Optics | Page hits: 162439 | Created: 01.2004 | Last update: 12.2013 |